VACCINE CLINIC INFORMATION
WILKES COUNTY HEALTH DEPT IS CURRENTLY OUT OF VACCINES.
Due to the 'High Need, Low Supply' status of the COVID-19 vaccine right now, please know that we are only able to distribute the vaccine in the order that is in accordance with the Vaccine Distribution Prioritization Framework given to us by the State of North Carolina. While this is can be frustrating, we are doing everything we can to get the vaccine out those in the current phase. Please be patient with staff and volunteers as we are all fighting this fight together.
The Wilkes County Public Health Department is on the front lines distributing the vaccine. Wilkes Health is committed to providing accurate and reliable information on the coronavirus, the best ways to prevent its spread and any approved and safe vaccine that is produced.
However, there are still many unanswered questions. This page will be updated as some of those questions are answered, but in the meantime, here is what we do know. To view how many people have received the vaccine in Wilkes County click here. To visit the NC DHHS webpage to read the latest information regarding the vaccine click here.
Vaccine Phase Info Graphic
In order to determine what phase you are in, please see the info graphic below.
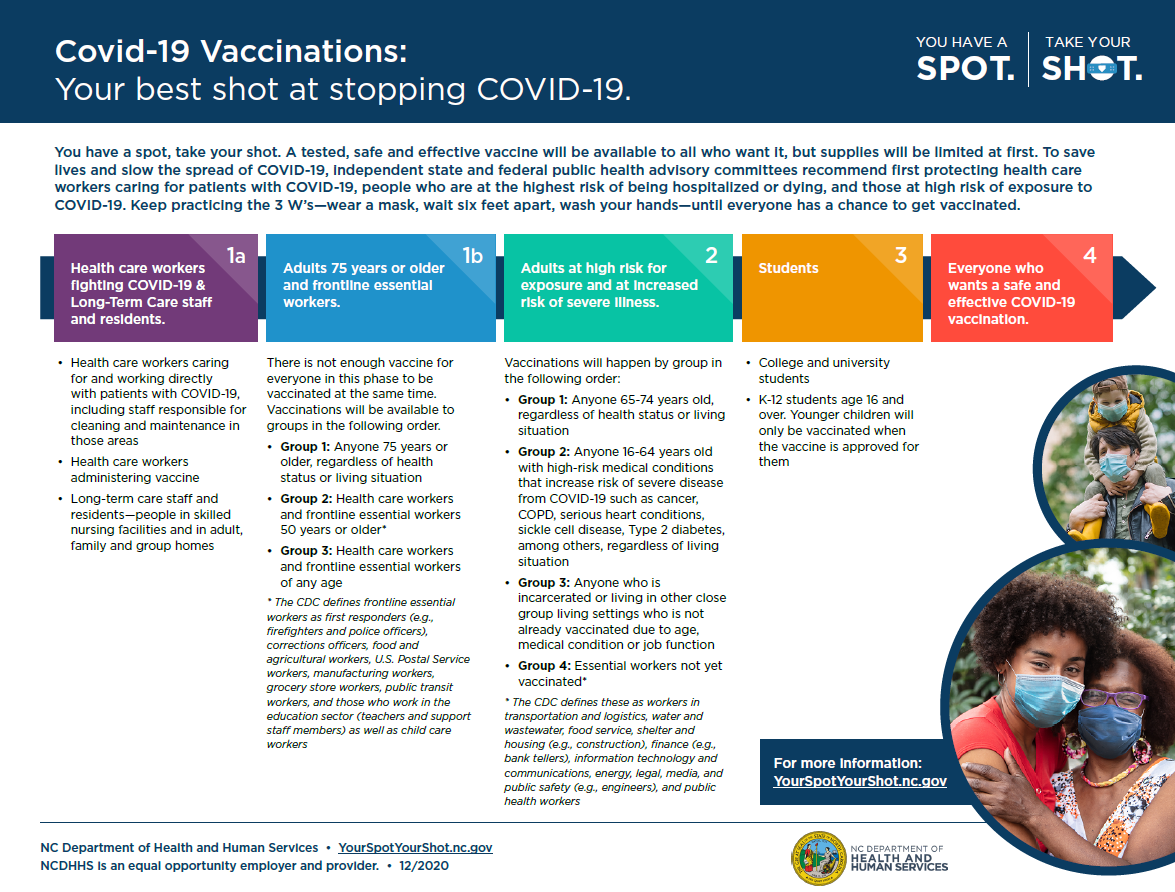
Vaccine Safety & Development
What all goes into developing a vaccine?
The COVID-19 vaccine development process is similar to other vaccines. Private companies work on creating a vaccine by conducting clinical trials to determine efficacy and safety of various vaccines. The vaccines are then given authorization for use by the U.S. Food and Drug Administration. In an opinion column for USA Today in October, Dr. Peter Marks, the director of the Center for Biologics Evaluation and Research at the FDA, wrote that “whether a vaccine is made available through an EUA [Emergency Use Authorization] or through a traditional approval, FDA will ensure that it is safe and effective.”
What does the vaccine include and how does it work?
More specific information will be shared whenever a vaccine is approved by the FDA and is ready for distribution.
According to the CDC, "COVID-19 vaccines help our bodies develop immunity to the virus that causes COVID-19 without us having to get the illness. Different types of vaccines work in different ways to offer protection, but with all types of vaccines, the body is left with a supply of 'memory' T-lymphocytes [that attach infected cells] as well as B-lymphocytes that will remember how to fight that virus in the future."
For more, visit the CDC's page on how the COVID-19 vaccine will work by clicking here.
How do we know the vaccine is safe? Are there any side effects?
More information will be shared whenever a vaccine is approved by the FDA and is ready for distribution. As stated above, every approved vaccine will undergo clinical trials to determine efficacy and safety.
For more, visit the CDC's page on COVID-19 vaccine safety by clicking here.
Vaccine Timeline
What is the current status of vaccine development?
There are many vaccines in different stages of development around the world. Vaccines go through three phases of testing: Phase 1 (safety and dosage testing), Phase 2 (expanded safety trials) and Phase 3 (large-scale efficacy testing). These stages of testing are very important to make sure that vaccines will be effective and that they will not have dangerous side effects. Because so many people will be taking a potential vaccine, it is important to minimize even rare negative side effects.
Multiple COVID-19 vaccines are under development. As of November 18, two vaccines trials have reported preliminary findings, with both offering promising results.
A vaccine developed by Pfizer reportedly was found to be more than 90% effective in preventing COVID-19 in participants who had not developed an infection and did not cause any safety concerns. Additionally, a vaccine developed by Moderna was found to be 94.5% effective in preliminary trials.
Regularly check the website for the Centers for Disease Control and Prevention for the latest information about COVID.
When will the vaccine be ready?
The goal for Operation Warp Speed is to deliver safe vaccines that work, with the first supply potentially becoming available before the end of 2020. When a vaccine is authorized or approved in the United States, there may not be enough doses available for all adults. Supplies will increase over time, and all adults should be able to get vaccinated later in 2021. However, a COVID-19 vaccine may not be available for young children until more studies are completed.
A major step to making a vaccine ready is the issuance of an Emergency Use Authorization (EUA). An Emergency Use Authorization (EUA) is issued by the Food and Drug Administration (FDA) during a public health emergency to allow the use of new medical products, such as a vaccine, more quickly. An EUA requires the submission of data that demonstrates a vaccine’s safety and that it can prevent disease. Before issuing an EUA for a COVID-19 vaccine an independent advisory committee will review the vaccine testing data. The Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices will review the
data and recommend who should be vaccinated based on clinical trial results. For example, it may recommend that a vaccine only be used for a certain age group based on the results of clinical trials.
When can I expect to get a vaccine?
While no vaccine has been approved for use in the United States yet, public health workers around the country have started preparing for how to distribute vaccines to the public — including in Wilkes County.
The first priority in Wilkes County will be first responders and healthcare workers, specifically those administering vaccines and staff at long-term care facilities. The next targeted group will be adults that have certain chronic health conditions. A county-level vaccine distribution plan will be made publicly available in the near future.
It may take a few months for enough doses to be made and distributed for everyone. It is also important to note that the vaccine will likely come in two doses, which will be given several weeks apart.
Vaccine Distribution
Who will get the vaccine first?
According to the North Carolina Interim COVID-19 Vaccination Plan, released by the state government on October 16, the populations prioritized by the state are "healthcare workers at high risk of exposure, essential workers (e.g., emergency management, fire, etc.), and long-term care staff and residents. These vaccines will most likely be administered in closed settings for employees or residents. Further, we are prioritizing populations with high risk of morbidity and mortality of COVID-19, especially those in settings or occupations with increased risk of exposure, e.g., people living in congregate settings (migrant farm workers, incarcerated people, people in homeless shelters), workers in high-density occupation and frontline workers."
According to the NCDHHS plan, early doses of the vaccine are likely to be shipped in increments of 1,000 due to vaccine storage needs at ultra-cold conditions, but that is not a guarantee of how many doses Wilkes County will receive at the beginning of the distribution phase.
Does everybody get the same vaccine?
More information will be shared whenever a vaccine is approved by the FDA and is ready for distribution.
What will the vaccine cost?
The COVID-19 vaccine will be available at no cost to everyone, no matter whether an individual has health insurance. The federal government will be purchasing the vaccines.
Vaccine Concerns
How do I know I can trust this vaccine?
We know many have questions about the safety and effectiveness of the COVID-19 vaccine. That’s one of the reasons we’ve constructed this page – to share information and updates with you. As noted above, vaccine trials are underway to study both the safety and efficacy (how well they work) of vaccines. Through these trials, tens of thousands of Americans have received the vaccine, and doctors pay close attention to any side effects, as well as how well the vaccines work in preventing COVID-19.
These trials have also included diverse groups of participants from different backgrounds, ages, races, and regions. One of North Carolina’s guiding principles in its vaccine plan is actively engaging and drawing upon the experience and expertise of leaders of different backgrounds, including historically marginalized populations.
Before vaccines are available to the broader public, they must go through this research process and receive approval from the US Food and Drug Administration (FDA). And while many are eager for this process to be finished, it is important and necessary to ensure the vaccine can be trusted.
Why do we even need a vaccine?
Vaccines have played a key role in eliminating or nearly eliminating many deadly diseases around the world. Multiple strains of polio that once wreaked havoc on children across America are now virtually eliminated due to a vaccine. Other diseases like smallpox and rubella met similar fates in the United States due to successful vaccination development and distribution.
Public health experts have said from the beginning of the pandemic that, as well as being the most significant tool, once developed, to curbing the spread of the coronavirus, a successful and effective vaccine will allow for the easing of restrictions and a resumption of “normalcy.” While there is no timeline for those actions, the more people take the vaccine, the closer we get to “normal.”
If I get a vaccine, does that mean I can go back to “normal” immediately – like not wearing a face covering and stopping social distancing?
No. While experts learn more about the protection that COVID-19 vaccines provide under real-life conditions, it will be important for everyone to continue using all the tools available to us to help stop this pandemic, like covering your mouth and nose with a mask, washing hands often, and staying at least 6 feet away from others. Together, COVID-19 vaccination and following CDC’s recommendations for how to protect yourself and others will offer the best protection from getting and spreading COVID-19. Experts need to understand more about the protection that COVID-19 vaccines provide before deciding to change recommendations on steps everyone should take to slow the spread of the virus that causes COVID-19. Other factors, including how many people get vaccinated and how the virus is spreading in communities, will also affect this decision.
More Resources: